About Neornat
CEO Message
Company Identity
NEORNAT Inc. develops first-in-class anti-cancer RNA drugs targeting functional RNAs and their specific regulators in cancer.


- The New RNA Therapeutics Company
- First-in-Class Target Therapeutics
RNA Therapeutics
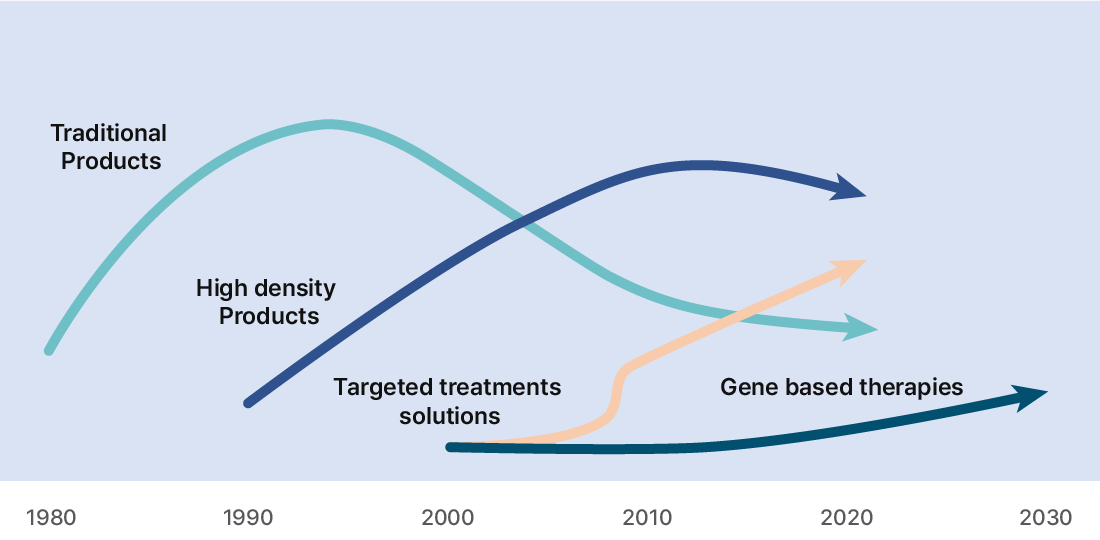
The need for RNA therapy
- As a next-generation genetic drug, sufficient potential has been verified through the COVID-19 vaccine.
- Normalizes intracellular pathological mechanisms that are difficult to target with existing drugs.
- With the development of RNA stability and delivery technology, many obstacles for the development of RNA therapeutics are being resolved.
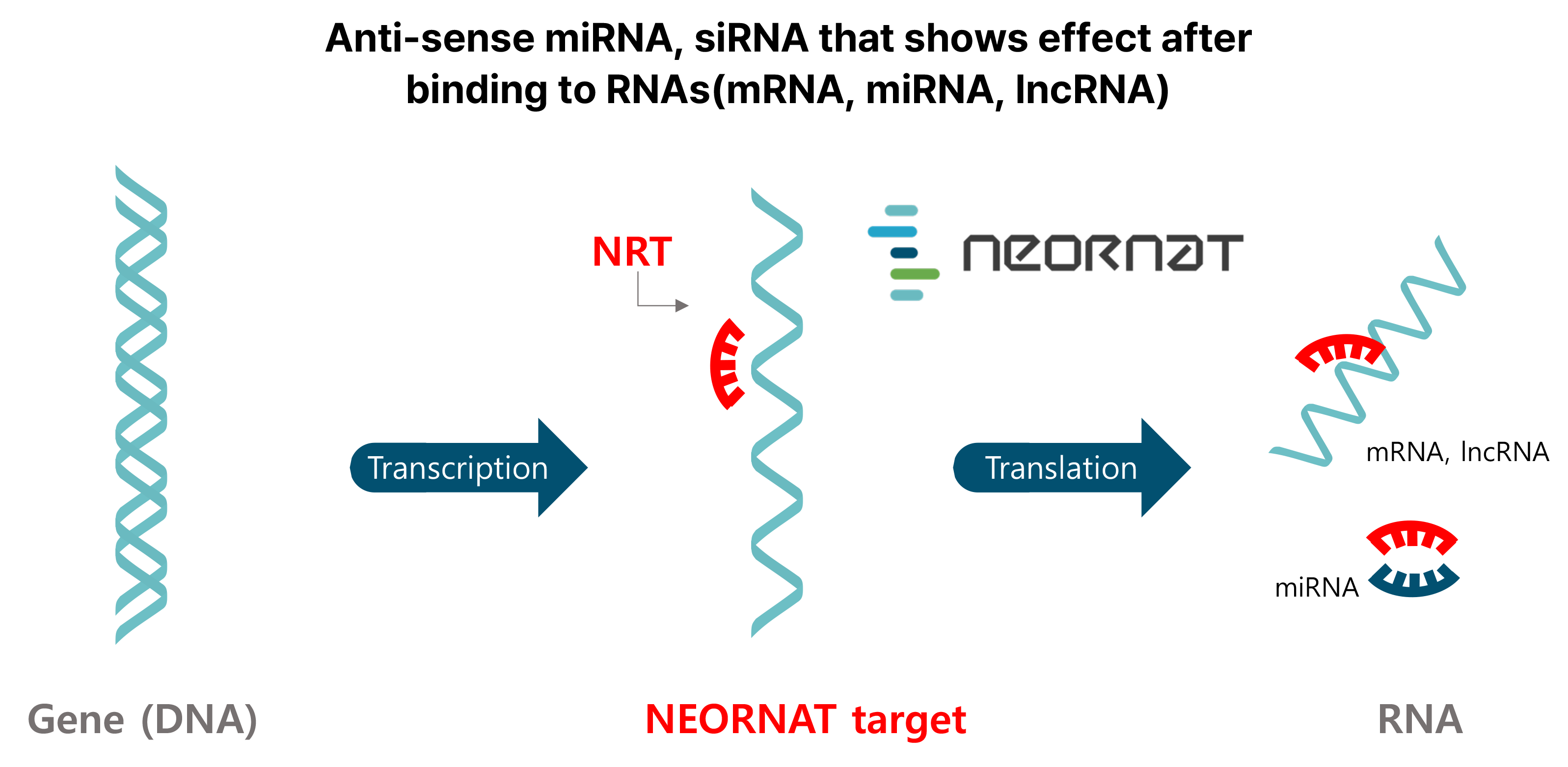
Advantages of RNA Therapeutics
- It fundamentally cures diseases by blocking disease-expressing genes.
- As a substance originally present in the body, it is not toxic by itself, and unlike DNA therapy, it does not affect the host genome, so there is little concern about safety.
- The production process is relatively simple and inexpensive.
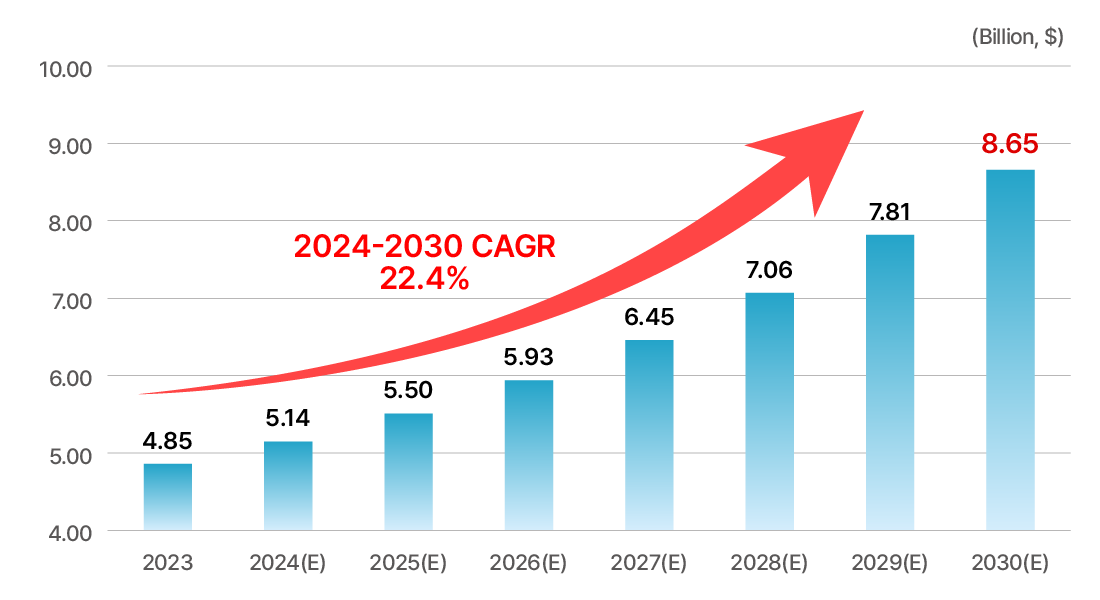
Rapid growth of the RNA therapeutics market
- The global market is showing high growth every year as siRNA treatment was approved by the FDA in 2018.
- As a first-in-class new drug, active technology export is possible even in the early stages of development.
History
2025
-
07
Entered into a strategic MOU for business development with MediRama Co., Ltd.
-
06
NEORNAT, Co., Ltd. Submitted a pre-IND review application for NRT-YHD_001 to the Ministry of Food and Drug Safety (MFDS)
-
06
NEORNAT, Co., Ltd. Participated in Bio International (BIO USA) 2025 and held partnering sessions with global pharmaceutical companies
-
06
NEORNAT, Co., Ltd. Launched a collaborative R&D project with Certest to develop an RNA-based gene therapy for lung cancer, NRT-YHD_002
-
05
Selected for the 2025 Startup-Centered University (Pre-Startup) Program by KISED, Ministry of SMEs and Startups
-
04
NEORNAT, Co., Ltd. Presented research at the AACR Annual Meeting 2025 (American Association for Cancer Research)
-
03
NEORNAT, Co., Ltd. Held the 5th Annual General Meeting of Shareholders
-
01
Completed pre-IND consultation with the Ministry of Food and Drug Safety (MFDS) for NRT-YHD_001
-
01
NEORNAT, Co., Ltd. Signed a memorandum of understanding (MOU) with Beyond Bridge & Co. for technology transfer and investment collaboration
2024
-
11
NEORNAT, Co., Ltd. Participated in Bio Europe-Fall 2024 and held partnering sessions with global pharmaceutical companies
-
06
Selected for the Post-TIPS (POST-TIPS) Program by the Korea Business Angels Association (KBAA)
-
06
CEO Suk Woo Nam invited as a panel speaker at the 1st Korea Industrial Pharmacists Conference
-
05
NEORNAT, Co., Ltd. Presented at BioKorea 2024 in the special session on Next-Generation Drug Platform Development Strategies
-
05
NEORNAT, Co., Ltd. Selected to participate in the Scale-up Plaza IR Competition Program organized by the Ministry of SMEs and Startups, recognizing the company’s technological excellence
-
05
NEORNAT, Co., Ltd. Held a partnering meeting with C Cube Lab for global technology transfer collaboration
-
04
NEORNAT, Co., Ltd. Presented research at the AACR Annual Meeting 2024 (American Association for Cancer Research)
-
03
Selected for the Global Co-Development and Technology Transfer Support Program by the Korea Drug Development Fund (KDDF)
-
01
NEORNAT, Co., Ltd. Participated in Biotech Showcase 2024 and conducted global partnering meetings
2023
-
12
NEORNAT, Co., Ltd. completes investment attraction from Korea Technology Finance Corporation
-
12
NEORNAT Co., Ltd. signed an agreement with Liberi Group, a global consulting company, to transfer overseas technology and promote multinational joint development of NEORNAT's major assets.
-
11
Visit and inspection of WuxiAppTech, Neona Co., Ltd.’s IND carrying out CDMO agency
-
07
Signed a joint R&D agreement with Neona Co., Ltd. and Ceratgen Co., Ltd. for 'RNA-based liver cancer immunotherapy'
-
05
[Korea Angel Investment Association] Selected as a TIPS startup commercialization support project (US$ 80,000)
-
04
NEORNAT Co., Ltd. presents NRT-YHD_001 pipeline non-clinical results at the 2023 AACR at Florida, Olando, USA
-
04
National New Drug Development Agency, KDDF] New drug development project (non-clinical development part) final selection (support size, US$1.6 million ( / 2 years)
Macrophage activity-based liver cancer RNA immunotherapy NRT-YHD_001 IND Package completion and approval goal -
02
Signed a business agreement for IND approval of RNA-based anti-cancer drug NRT-YHD_001 with AIMSBiosciences Co., Ltd.
2022
-
11
Selected as Korea Angel Investment TIPS
-
09
Established NEORNAT affiliated R&D Center (2F, 47-3, Banpo-daero 39-gil, Seocho-gu, seoul, 06579 Korea )
-
06
Steintech Bio Season 1, Final Round Top 5 Selection and Best Company Selection
-
03
Completed pre-round Series A investment
-
02
Signed an MOU with Labgenomic for research and development of RNA-based anti-cancer drugs
2021
-
12
Catholic University Industry-University Cooperation Foundation- NEORNAT Inc. transfers 3 technologies for new drug development
-
12
NEORAT - AlgokBio signed an agreement to be the preferred bidder for liver cancer drug development
-
07
Selected as Korea Angel Investment TIPS
-
04
Selected as a 3-year project for the disease-centered translational research project
-
01
Shinhan Capital Investment Attraction
2020
-
11
Catholic University Industry-University Cooperation Foundation - Completed technology transfer for 4 cases of NEORNAT Inc.
-
10
Selected as K-BIC STAR (New drug development start-up company, Korea Health Industry Promotion Agency)
-
09
Completed attracting angel investment
Established NEORNAT Inc.
People

Founder, CEO & CTO Suk Woo Nam
- Professor, Department of Pathology, College of Medicine, The Catholic University of Korea (present)
- Director of Functional Rnomics Research Center, Catholic University of Korea (present)
- President, Basic Professors Association of College of Medicine, The Catholic University of Korea (Present)
- Sungkyunkwan University Department of Pharmacy, Master of Pharmacy, Doctor of Pharmacy
- Post-doctoral Fellow, Lab of Pathology, NCI, NIH, USA
- Senior Scientist, Genome Institute of Singapore, Singapore
- Published more than 254 SCI papers, and 28 patent applications and registrations
- Korea National Academy of Medicine Award for Basic Medicine (Pfizer Medical Award), 2014
- Minister of Health and Welfare Award, 2015
- Minister of Environment Award, 2013
- Served as a judge for the Ho-Am Award for Medicine
- Served as a judge for KOSDAQ technology special listing (RNA new drug part)

Head of NEORNAT Regulatory Science Chang Won Park, Ph.D.
- Pharmaceutical Regulatory Science General Manager
- Ph.D. in Pharmacy, Department of Pharmacy, Sungkyunkwan University, Korea.
-
Former Health Researcher at the Ministry of Food and Drug Safety
Experience in various departments including General Toxicology, Neurotoxicology, Organ System Drugs, Antibiotic and Anticancer Drugs, Drug Approval Review Coordination, Hazardous Substance Analysis, Cosmetic Research, Oncology Drugs, Pharmacology Research, Cardiovascular and Imaging Devices, and Medical Device Research.
- Recipient of the Prime Minister's Commendation from the Ministry of the Interior and Safety, 2009.
- Expert in Non-clinical Drug Testing (Toxicology Testing)
- Expert in the Safety/Efficacy Evaluation of Drugs and Medical Devices

Head of Nonclinical Development Sang Yean Kim
- Team leader : non-clinical development of new drug candidates department (current)
- Ph.D., College of Medicine, The Catholic University of Korea
- Major in Tumor Biology
- Major in RNA Cancer Biology
- Major in Targeted-RNA therapy Development
- 7 SCI articles including Oncogene and 3 patent applications
- Modified mouse lung cancer analysis expert
- Responsible for the development of Macrophage RNA Immunotherapy
- Responsible for research and development of siRNA mix therapeutics
- Responsible for GLP-Tox and PK/PD assessment

Head of active and lead material development Min Jeong Na
- Team leader: new drug effective and leading substance development & verification department (current)
- Ph.D., College of Medicine, The Catholic University of Korea
- Major in Tumor Biology
- Major in DNA Repair & Cancer Biology
- Major in RNA Variation & Cancer
- 4 SCI articles including Experimental Molecular Medicine and 3 patent applications
- Real-time mouse liver cancer image analysis expert
- Responsible for the development of novel DNA repair enzyme-targeted therapy
- Responsible for non-clinical efficacy evaluation

Head of early cancer diagnosis development Jung Hwan Yoon
- Team leader: digestive cancer RNA drug development (current)
- Ph.D., College of Medicine, The Catholic University of Korea
- Major in Tumor Biology
- Experience in the early diagnosis and development of gastric cancer
- 50 SCI articles including Cancer Commun and 13 patent applications
- Gastric cancer early diagnosis kit development expert
- Specialist in the development of anticancer drugs targeting gastric cancer carcinogenesis
- Head of gastric cancer drug effective and lead material development and verification research
Collaborator
Domestic
Overseas
Contact Us
Headquarter
-
Address
2F, 47-3, Banpo-daero 39-gil, Seocho-gu, Seoul (06579)
- TEL
-
FAX
02-595-2810
Research Center
-
Address
3F, 47-3, Banpo-daero 39-gil, Seocho-gu, Seoul (06579)
- TEL
-
FAX
02-595-2810













